SANUM-KEHLBECK GmbH & Co. KG
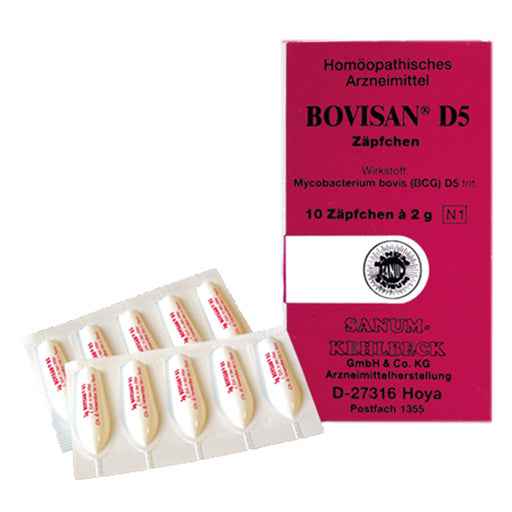
BOVISAN D 5 suppositories
BOVISAN D 5 suppositories
Couldn't load pickup availability
BOVISAN D 5 suppositories
Homeopathic medicine
active ingredients
- 200 mg Mycobacterium bovis D5
excipients
- hard fat
Important information (mandatory information):
Bovisan D5 suppositories. Areas of application: Registered homeopathic medicine, therefore no therapeutic indication is given.
For risks and side effects, read the package insert and ask your doctor or pharmacist.
INSTRUCTIONS FOR USE: INFORMATION FOR THE USER
Bovisan D5 suppositories
Homeopathic medicine
Active ingredient: Mycobacterium bovis (BCG) e muribus cellulae (lyophil., sterile.) Trit. D5
Dear patient,
Please read this leaflet carefully because it contains important information about what you should bear in mind when using this medicine. If you have any questions, contact your doctor or pharmacist.
Areas of application:
Registered homeopathic medicine, therefore no therapeutic indication is given. Note to the user: If symptoms of illness persist while using the medicine, medical advice should be sought.
Contraindications:
When should you not use Bovisan D5?
Do not use if:
- autoimmune diseases.
- Children under 12 years of age.
- pregnant and breastfeeding women.
- known hypersensitivity to Mycobacterium bovis.
Precautions for use and warnings:
None known.
Interactions with other drugs:
Other immunosuppressive drugs can impair the effectiveness of Bovisan D5. An interval of four weeks should be observed before and after treatment with orally administered live vaccines. General note: The effect of a homeopathic medicine can be adversely affected by generally harmful lifestyle factors and by stimulants and stimulants. If you are taking other medicines, ask your doctor.
Type and duration of use:
The following information applies unless your doctor has prescribed otherwise. Please follow the instructions for use, otherwise this medicine will not work properly!
How long should you use Bovisan D5?
Bovisan D5 should be discontinued after a maximum of 4 weeks of treatment. If the symptoms improve, the frequency of use should be reduced. Homeopathic medicines should also not be used for long periods without medical advice.
Side effects:
Due to the content of specific organic components in Bovisan D5, hypersensitivity reactions, mainly in the form of skin reactions, can occur in rare cases and an allergy to the component Mycobacterium bovis can be triggered. The medicine should then be discontinued and a doctor consulted. Note: When using a homeopathic medicine, the existing symptoms can temporarily worsen (initial worsening). In this case, you should stop taking the medicine and consult your doctor.
Reporting of side effects:
If you notice any side effects, contact your doctor or pharmacist. This also applies to side effects not listed in this leaflet. You can also report side effects directly to the Federal Institute for Drugs and Medical Devices, Dept. Pharmacovigilance, Kurt-Georg-Kiesinger-Allee 3, D-53175 Bonn, website: www.bfarm.de. By reporting side effects, you can help provide more information about the safety of this medicine.
Notes and information on the shelf life of the medicine:
The expiry date is printed on the suppository strip and the outer packaging. Do not use this pack after this date! Do not store above 25°C. Never dispose of the medicine via wastewater (e.g. not via the toilet or sink). Ask your pharmacist how to dispose of the medicine when you no longer use it. This will help protect the environment. You can find more information at www.bfarm.de/arzneimittelentsorgung.
Composition:
1 suppository contains the active ingredient: 0.2 g Mycobacterium bovis (BCG) e muribus cellulae (lyophil., sterile.) Trit. D5 (HAB, regulation 6). Other ingredients: hard fat.
Dosage form and content:
10 and 100 suppositories of 2 g each for rectal use
Pharmaceutical company and manufacturer:
Sanum-Kehlbeck GmbH & Co. KG
Postfach 1355
D-27316 Hoya
This leaflet was last revised in June 2018.
Source: Information in the package leaflet
as of: 08/2022Areas of application: Registered homeopathic medicinal product, therefore without specification of a therapeutic indication.
Share